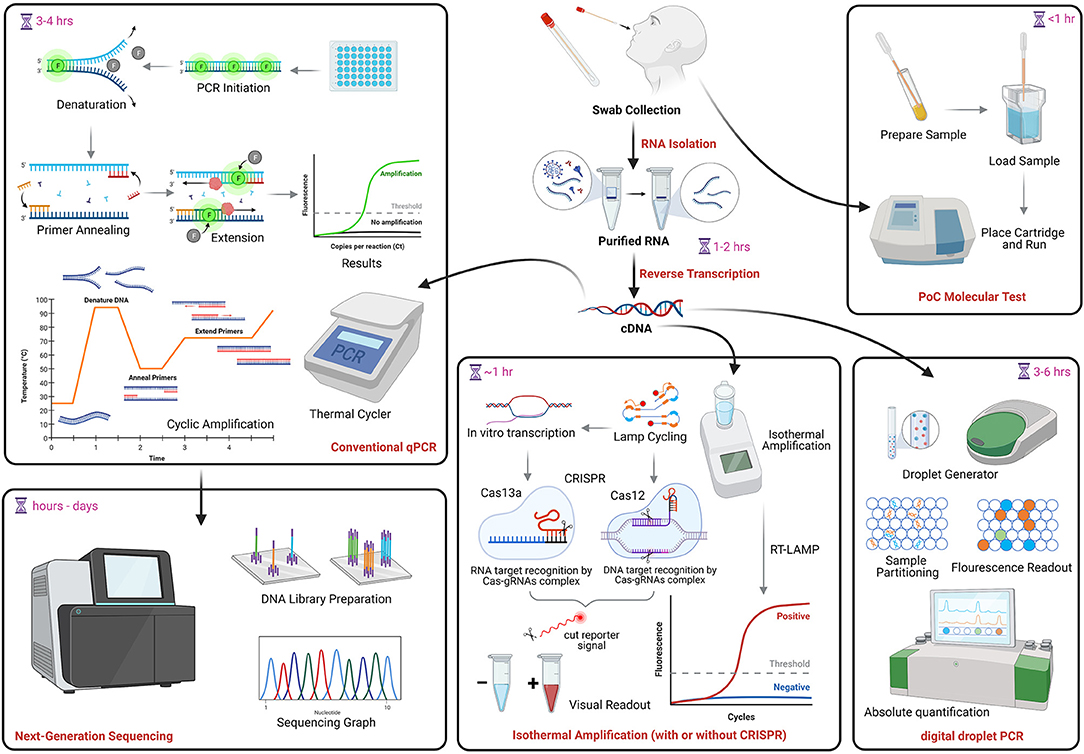
Cobas® Liat® Pcr System
Cobas® liat® pcr system. The cobas Liat System brings lab-quality testing to the point of care delivering accurate results in 20 minutes or less enabling you to make the best. The high PCR sensitivity and specificity achieved with the cobas Liat System enables patients and clinicians to have reassurance in their diagnostic results at all points of care in 20 minutes or less across a growing menu of assays. The cobas SARS-CoV-2 Influenza AB Nucleic acid test for use on the cobas Liat System cobas SARS-CoV-2 Influenza AB is an automated multiplex real-time RT-PCR assay intended for the.
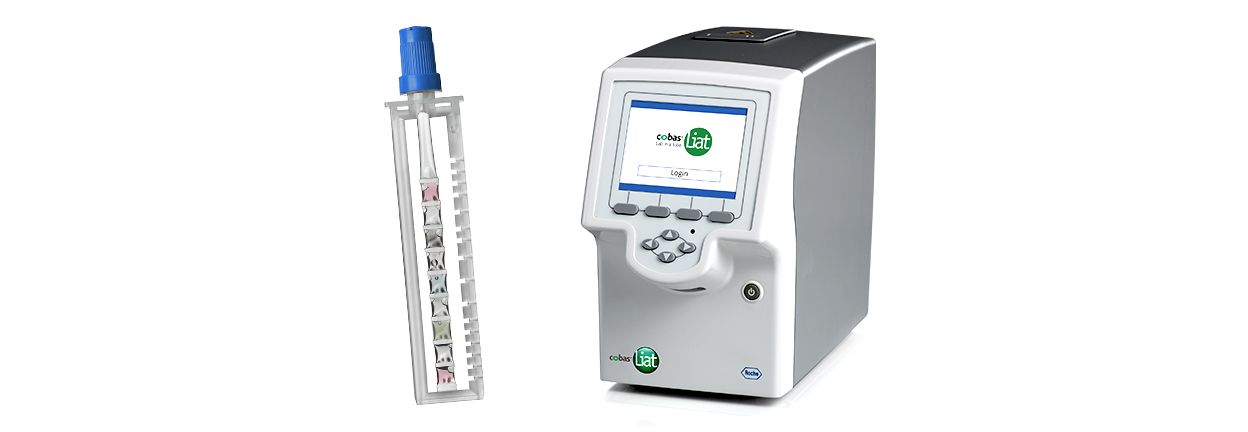
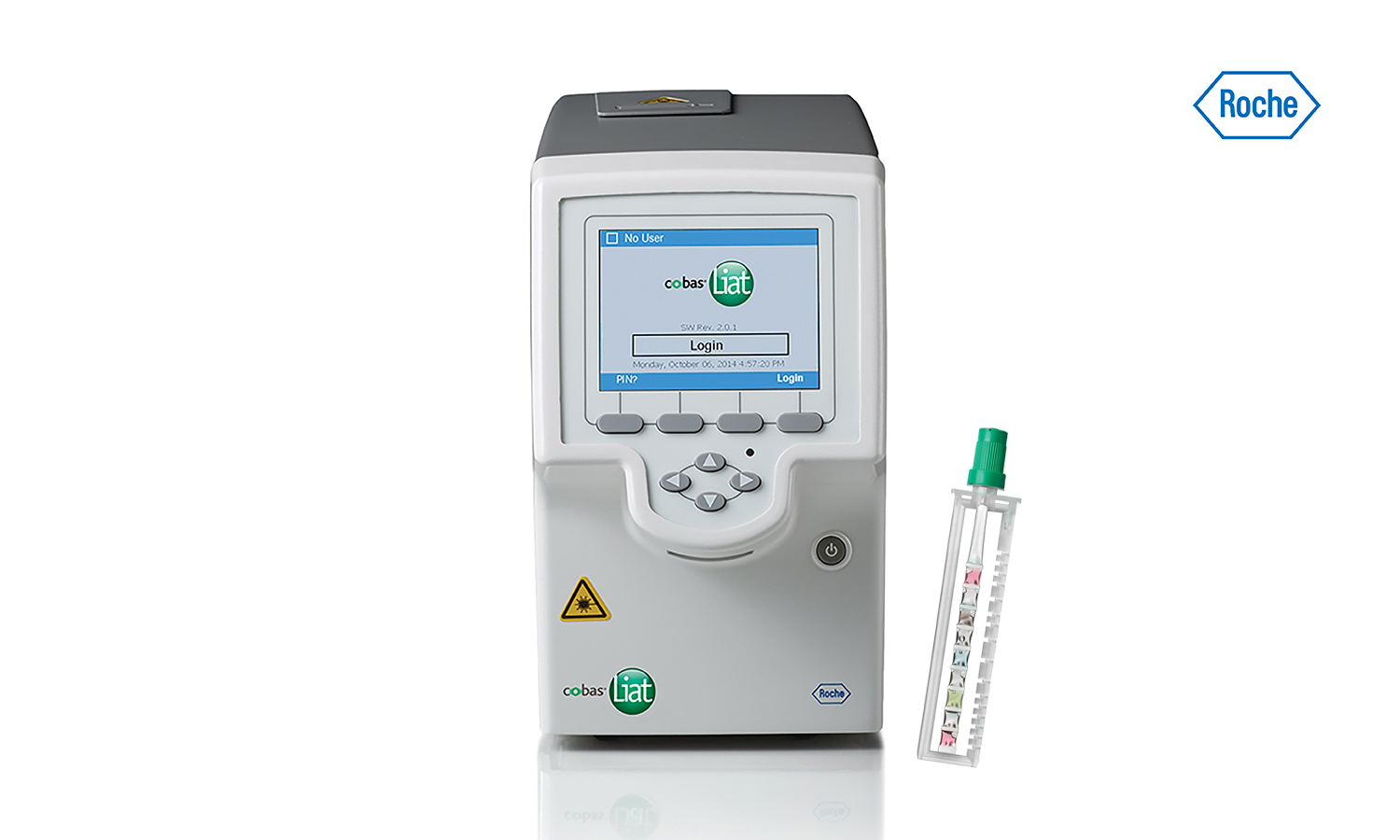
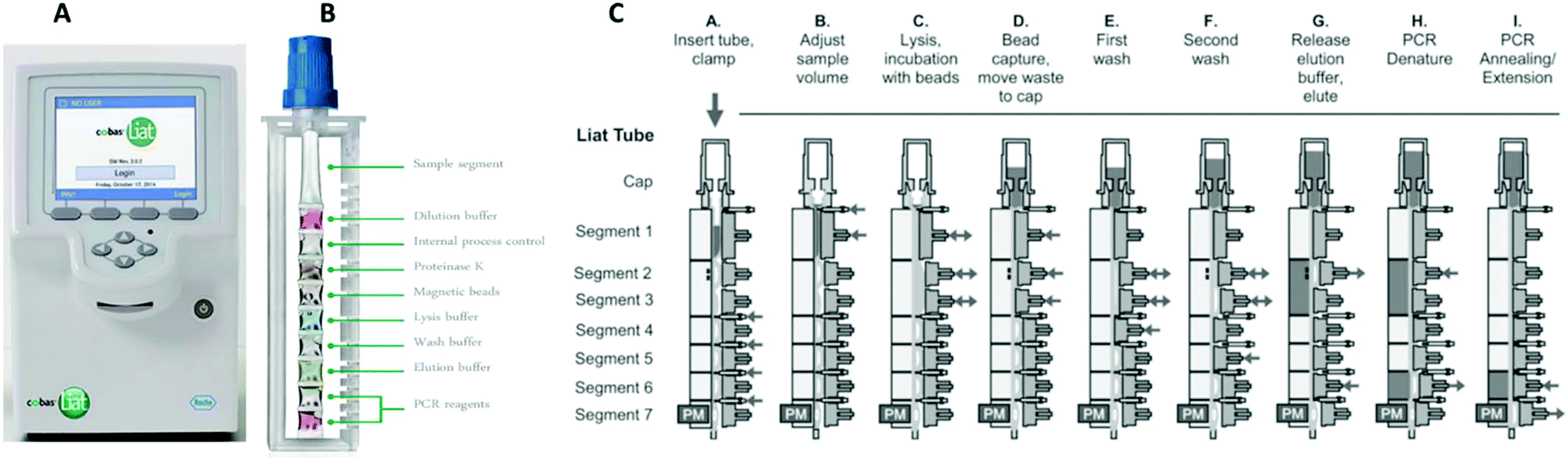
Cobas Liat PCR System Technical Specifications Actual size cobas Liat Analyzer and assay tube shown Closed system minimizes contamination. The system includes a small benchtop analyzer safeguarded by an extensive array of instrument controls and assay tubes tailor-made for specific targets. The total point-of-care PCR solution.
This video summarizes the compellin. The cobas Liat System generates high-quality polymerase chain reaction PCR results in turnaround times of 20 minutes or less across a growing menu of assays. Sign up today to get the latest news and updates on the cobas Liat PCR System.
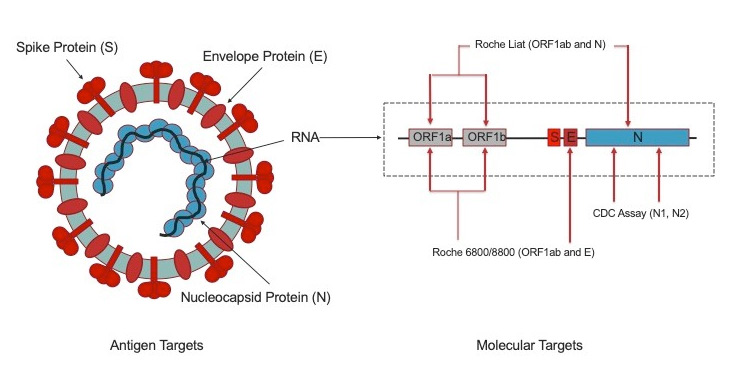
In contrast the Cobas Liat detected all 44 100 samples. The cobas Liat System brings real-time answers for patients and their caregivers with the assurances of PCR technology in 20 minutes or less for all assays in the portfolio. The cobas SARS-CoV-2 Influenza AB Nucleic acid test for use on the cobas Liat System cobas SARS-CoV-2 Influenza AB is an automated multiplex real-time RT-PCR assay intended for the simultaneous rapid in vitro qualitative detection and differentiation of SARS-CoV-2 influenza A and influenza B virus RNA in healthcare provider-collected nasopharyngeal and nasal swabs and self.
The Cobas Liat PCR System is small easy to use and fastable to provide accurate results in the point-of-care. The expanded menu of assays for use on the cobas Liat System includes the respiratory portfolio of cobas Strep A cobas Influenza AB cobas Influenza AB RSV. 68008800 Systems is a real-time RT-PCR test intended for the qualitative.
Detection of nucleic acids from SARS-CoV-2 in healthcare provider-instructed self-collected anterior nasal. The cobas Liat PCR System is small easy to use and fastable to provide accurate results by. The cobas Liat PCR System is a compact rapid molecular testing system for the detection of Influenza AB Influenza AB RSV and Strep A at the point of care.
The cobas Liat System is the only FDA-cleared molecular diagnostic platform to offer PCR results in real time the company said. Cobas SARS-CoV-2 09179909001-01EN Doc Rev.
Detection of nucleic acids from SARS-CoV-2 in healthcare provider-instructed self-collected anterior nasal.
The Cobas Liat PCR System is small easy to use and fastable to provide accurate results in the point-of-care. About the cobas Liat System. Detection of nucleic acids from SARS-CoV-2 in healthcare provider-instructed self-collected anterior nasal. Cobas Liat PCR System Technical Specifications Actual size cobas Liat Analyzer and assay tube shown Closed system minimizes contamination. The cobas Liat System brings this proven technology directly to your point of care and your patients. The Cobas Liat PCR System is small easy to use and fastable to provide accurate results in the point-of-care. Comparison of Cobas Liat ID NOW and Oxsed assays with reference RT-PCR. The cobas Liat System is the only FDA-cleared molecular diagnostic platform to offer PCR results in real time the company said. Sign up today to get the latest news and updates on the cobas Liat PCR System.
Definitive results are generated in 20 minutes or less to aid a treatment decision. Sign up today to get the latest news and updates on the cobas Liat PCR System. About the cobas Liat System. The system includes a small benchtop analyzer safeguarded by an extensive array of instrument controls and assay tubes tailor-made for specific targets. In contrast the Cobas Liat detected all 44 100 samples. Cobas SARS-CoV-2 09179909001-01EN Doc Rev. The expanded menu of assays for use on the cobas Liat System includes the respiratory portfolio of cobas Strep A cobas Influenza AB cobas Influenza AB RSV.


























Post a Comment for "Cobas® Liat® Pcr System"